A Broken Healthcare Paradigm – Is Pharmacogenomics a Viable Strategy?
By Blake Keller, PharmD
Chief Operating Officer, RxGenomix
Most of us are aware of the state of American healthcare today – we spend too much money on healthcare for too little result, and very often get frustrated along the way. And this hasn’t gone unnoticed by policy makers, payers and frustrated patients. Yet, somehow, it’s not getting fixed. In one area, though, there is hope.
As a pharmacist, my job is to make sure my patients are on the right medications at the right dosage, and at the right time. Until recently, there has been a lot of guesswork involved in that, but that’s been changing with pharmacogenomic testing. By looking at a few small components of an individual’s DNA, pharmacists and physicians can predict the drug gene interaction that determines whether or not particular medications will work for that individual. This starts getting to the basic premise of personalized medicine – treat the individual rather than the disease. All it takes to get started is a simple cheek swab and the desire for a better way of practicing medicine.
US-based health expenditure growth is expected to double in the next eight years, reaching nearly $6 trillion by 2027. 1 Spending increases for prescription drugs is projected to grow through 2027, on average accelerating by 5.6 percent, mostly a result of faster utilization growth, the report states. This includes factors such as efforts on the part of employers and insurers toward better medication adherence among those with chronic conditions and changing pharmacotherapy guidelines.
The Broken Paradigm
The chart below represents what most individuals encounter in today’s broken, ineffective, and inefficient healthcare system. A patient most often chooses to seek medical attention because they have an “issue”. Despite wellness initiatives and yearly physicals, there are still many patients that do not engage primary care providers until they are symptomatic and, by then, disease progression may already be well underway. Our primary care providers and specialists do a wonderful job running tests, taking histories, and making a definitive diagnosis.
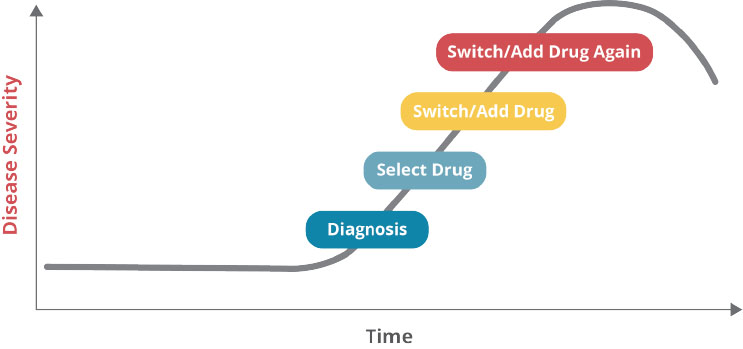
In the best situations, the provider engages the patient in a shared decision-making capacity to suggest lifestyle modifications, careful monitoring, and likely the addition of pharmacotherapy (medication) based on clinical guidelines. Good so far.
The patient starts taking the medication and, within one month, half of them will have either stopped taking the medication or aren’t taking it correctly. This is where the wheels come off the bus.
There are a myriad of reasons as to why this may be the case, including an unaffordable cost, adverse drug events popping up, or the patient could just be forgetting to take the medication. And if the condition is “silent”, the patient may simply feel there is no need for the medication and choose not to take it. These are just a few of the potential causes for non-adherence. Fast forward a month or two and the patient returns to their provider for a follow-up visit. At this point the provider is faced with a decision.
The lab results may not reflect improvement and the patient, if symptomatic, likely backs that up and piles their frustration on top of it. The condition continues to progress. The provider then decides to go ahead and change the medication, as they are uncertain if the original medication was working at all, and they might even add a second medication. And the whole thing starts over again. This maddening cycle of repeat visits, medication changes, and incomplete therapeutic response correlates with a worsening of the disease and the costs start piling up. Pharmacogenomic testing can interrupt or complete prevent this cycle of failure from ever happening and contribute to getting the patient on the right medication the first time.
A significant driver in poor therapeutic response is an individual’s genetics, specifically their pharmacogenes. There is a small set of genes responsible for an individual’s response to medications. Depending on these genes, the same medication that is beneficial for one individual could be entirely ineffective or even life-threatening for another. Without pharmacogenomic testing, you’re taking your chances on efficacy, the potential for adverse drug reactions and a delay in effective therapy.
The Basics of Pharmacogenomics
The human genome includes some 3 billion nucleotide base pairs in the DNA that constitutes our 25,000 to 30,000 genes. A number of these are pharmacogenomics proteins—drug receptors, drug targets, drug metabolizing enzymes, and drug transporters— that are important for accurate and effective medication therapy. These include, but aren’t limited to, the Cytochrome P450 enzymes—CYP2D6, CYP2C9, CYP2C19, CYP3A4/5, and others.
Other proteins, such as VKORC1 and SLCO1B1, represent drug targets and transporters, respectively. These proteins and others are critical in drug response and the application of pharmacogenomics in precision medicine. Variances in the activity of these enzymes may affect an individual’s pharmacokinetics (absorption, distribution, metabolism, and excretion), as well as the pharmacodynamics (what effect the medication has on the body) of each medication the individual is taking.
We now understand that certain medications may achieve supra-therapeutic blood levels based on altered genetics, which may lead to toxicities and adverse drug events. At the other end of the spectrum, some metabolic issues may lead to drugs not converting to the active form of the medication necessary to produce a clinical effect. Unfortunately, the patient receives little to no benefit here whatsoever. It is important to understand that these types of issues are not “edge cases”. The likelihood of a patient having at least one genetic variant in their Cytochrome P450 genes is as high as 95%. This begs the question as to why a provider would ever prescribe a medication that has a significant chance of not working. Yet, that is what happens most of the time.
Providers utilize diagnostic testing and imaging to ensure they have a care plan informed by as much information as is possible prior to beginning a procedure or starting a therapeutic treatment. Unfortunately, pharmacogenomic testing hasn’t found its place in the standard of care just yet. Many times, providers are simply unaware of pharmacogenomics in general, or perhaps they haven’t yet figured out how to add pharmacogenomics testing into their busy clinical workflow in an efficient way.
It is also important to know that clinical usage guidelines for most medications on the market today are very largely derived through large, population-level trials and studies. New medications are brought to market through clinical trials, also conducted across large populations. These studies detail the medications’ effectiveness, and their failings, and seem to accept a certain rate of failure in approving the medication for use. You’ve heard the TV commercials listing all the side effects a medication could have. This is all understandable and appropriate based on yesterday’s model of medication therapy, but we need to move past that. Patients are truly unique individuals and pharmacogenomic testing helps us treat them as such.
Applying Pharmacogenomics to Patient Care
Pharmacogenomics (PGx) elucidates through a simple cheek swab which medications may be appropriate and which may not be, for each discrete individual patient. The model below represents an optimized model that is based on upon effective utilization of pharmacogenomics by PGx-trained providers.
An optimized clinical model is such that the provider is empowered with the genetic profile of their patient. When pharmacogenomics data is available to providers, they are able to choose medications that not only follow clinical guidelines but are selected specifically for the patient based upon their DNA. This model eliminates the trial-and-error approach, compresses the time to therapeutic response, and reduces adverse drug events and toxicities that would be attributable to variability in genetic response.
The correct application of pharmacogenomics is still evolving. RxGenomix is unique in the industry as our model is pharmacist-led. We acknowledge the complexity of provider workflows. In most care settings, physicians, nurse practitioners, and physician assistants see far too many patients per hour. Most are frustrated with their electronic health record (EHR) solution and quite simply cannot add anything additional to their workflows. The reporting that comes with pharmacogenomics testing is long, complex, and may not integrate into EHRs without significant interoperability strategies. RxGenomix works with healthcare providers to harness the power of pharmacogenomics by engaging one of the most underutilized healthcare assets in the industry — pharmacists.
RxGenomix pharmacists are well-trained in the science of pharmacogenomics and how to apply genetic testing to medication use. Our pharmacy model partners with healthcare organizations that understand the clinical utility of pharmacogenomics, but are struggling with the data, analytics, and clinical workflows to optimize clinical outcomes. We support our pharmacy teams with robust clinical software that allows our pharmacists to produce simple, clinically actionable care plans for patients that are informed by not just pharmacogenomic factors, but also myriad other clinical and financial inputs.
RxGenomix firmly believes there will be a day where pharmacogenomic testing is ubiquitous. In a few years, you will likely carry a smartphone embedded with your health record that is capable of informing your care team of all of your current medications, current conditions, clinical histories, and your genetic profile that would allow any provider to check your genetic test for the best medications for you prior to prescribing any medication. That said, we are not there yet.
We must be thoughtful and financially responsible as we prove the utility of pharmacogenomics. RxGenomix exists to optimize the clinical utility and associated outcomes that come with treating each patient as an individual.
Quite simply, the pharmacogenomic “test”, in and of itself, lacks substantive value. The true opportunity to advance the quadruple aim through pharmacogenomics is only realized by deploying the right combination of science, education, analytics, technology, and care team providers. RxGenomix is excited to change the conversation as it relates to pharmacogenomics.
1- February 2019 report released by the Office of the Actuary at the Centers for Medicare and Medicaid Services.